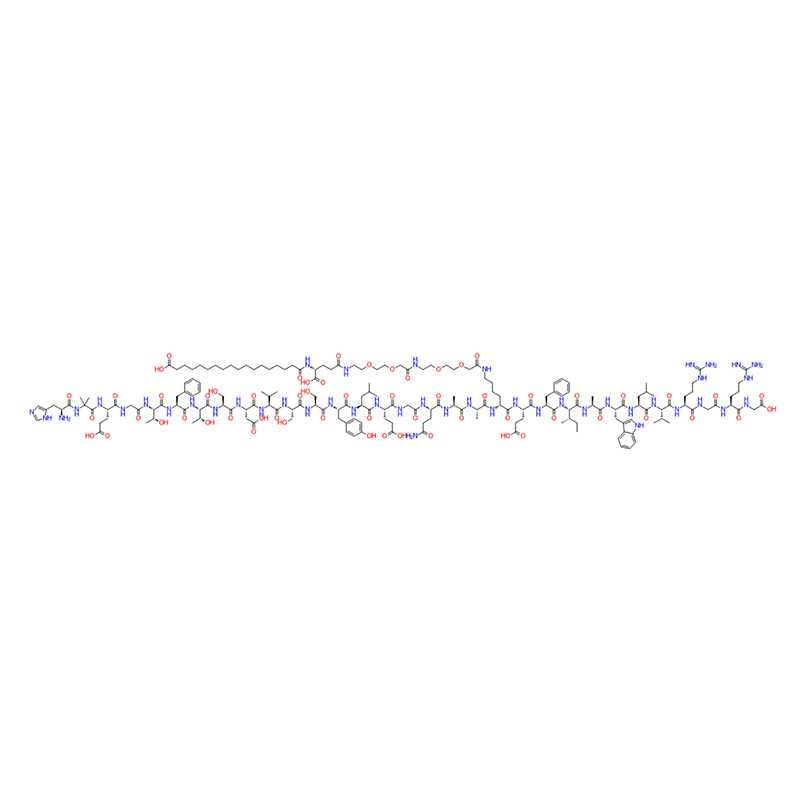
ʻO Semaglutide paha ka agonist GLP-1 maikaʻi loa.
I kēia manawa, ʻo nā lāʻau lapaʻau koʻikoʻi ma ka mākeke ka orlistat mai Roche, liraglutide mai Novo Nordisk a me semaglutide.
ʻO Wegovy, kahi analogue GLP-1 o Novo Nordisk, ua ʻae ʻia e ka FDA ma 2017 e mālama i ka maʻi maʻi type 2.I Iune 2021, ua ʻae ka FDA i ka hōʻailona slimming o Wegovy.
I ka makahiki 2022, ʻo ka makahiki hoʻolaha mua loa ma hope o ka papa inoa o Wegovy, ua loaʻa iā Wegovy he $877 miliona i nā hōʻailona hoʻemi kaumaha.
Me ka papa inoa o ka semaglutide, ʻo ka hoʻokele subcutaneous i hoʻokahi manawa i ka pule ua hoʻomaikaʻi nui i ka hoʻokō ʻana o nā maʻi, a ʻike ʻia ka hopena o ke kaumaha.ʻO ka hopena o ke kaumaha ma 68 mau pule he 12.5% kiʻekiʻe aʻe ma mua o ka placebo (14.9% vs 2.4%), a ua lilo ia i huahana hōkū i ka mākeke pohō kaumaha no kekahi manawa.
I ka hapaha mua o 2023, ua loaʻa ʻo Wegovy i ka loaʻa kālā o 670 miliona US kālā, ʻoi aku ka 225% i kēlā me kēia makahiki.
ʻO ka ʻae ʻia o ka hōʻailona kaumaha o ka semaglutide e hoʻokumu nui ʻia ma kahi haʻawina III i kapa ʻia ʻo STEP.ʻO ka haʻawina STEP ka loiloi nui i ka hopena therapeutic o ka subcutaneous injection o semaglutide 2.4mg i hoʻokahi pule i hoʻohālikelike ʻia me kahi placebo i nā mea maʻi obese.



Ua hoʻokomo ʻia ka haʻawina STEP i kekahi mau hoʻāʻo, kahi i loaʻa ai ma kahi o 4,500 mau maʻi maʻi ʻoi aku ke kaumaha a paʻakikī paha, me:
ʻO ka haʻawina STEP 1 (kōkua i ka nohona ola) hoʻohālikelike i ka palekana 68-wiki a me ka maikaʻi o ka subcutaneous injection o semaglutide 2.4mg i hoʻokahi pule me kahi placebo i ka makahiki 1961 obese a overweight paha nā pākeke.
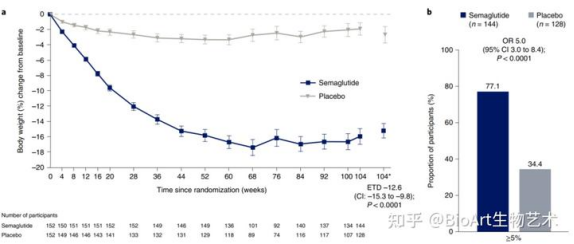
Ua hōʻike nā hopena i ka hoʻololi awelika o ke kaumaha o ke kino he 14.9% i ka hui semaglutide a me 2.4% i ka hui PBO.Ke hoʻohālikelike ʻia me ka PBO, ʻoi aku ka maʻamau o nā hopena ʻaoʻao o ka maʻi gastrointestinal o semaglutide, akā ʻo ka hapa nui o lākou he transient a hiki ke hoʻohaʻahaʻa me ka ʻole o ka hoʻomaha mau ʻana i ke ʻano o ka mālama ʻana a i ʻole ke koi ʻana i nā mea maʻi e haʻalele i ke aʻo ʻana.Hōʻike ka noiʻi STEP1 he hopena maikaʻi ke kaumaha o ka semaglutide i nā poʻe maʻi obese.
ʻO ka haʻawina STEP 2 (nā poʻe maʻi me ka maʻi diabetes type 2) hoʻohālikelike i ka palekana a me ka maikaʻi o ka subcutaneous injection o semaglutide 2.4 mg hoʻokahi manawa i ka pule me kahi placebo a me semaglutide 1.0mg i 1210 obese a i ʻole ke kaumaha nui no nā pule he 68.
Ua hōʻike ʻia nā hopena i hoʻololi nui ʻia nā manaʻo kaumaha o ke kino o nā hui lapaʻau ʻekolu, me -9.6% i ka hoʻohana ʻana i ka 2.4 mg o semaglutide, -7% i ka hoʻohana ʻana i ka 1.0mg o semaglutide, a me -3.4% i ka wā e hoʻohana ai i ka PBO.Hōʻike ka noiʻi STEP2 e hōʻike ana ka semaglutide i ka hopena maikaʻi o ke kaumaha no nā poʻe maʻi me ka maʻi diabetes type 2.
STEP 3 study (adjuvant intensive behavioral therapy) hoʻohālikelike i ka ʻokoʻa 68-week i ka palekana a me ka pono ma waena o ka subcutaneous injection o semaglutide 2.4 mg hoʻokahi manawa i ka pule a me kahi placebo i hui pū ʻia me ka intensive behavioral therapy i 611 obese a i ʻole ke kaumaha nui.
I nā pule he 8 mua o ke aʻo ʻana, ua loaʻa i nā kumuhana āpau ka meaʻai haʻahaʻa haʻahaʻa haʻahaʻa haʻahaʻa haʻahaʻa a me ka hoʻomaʻamaʻa kino koʻikoʻi i loko o ka papahana 68-wiki.Pono nā mea komo e hana i 100 mau minuke o ka hoʻoikaika kino i kēlā me kēia pule, me ka piʻi ʻana o 25 mau minuke i kēlā me kēia pule ʻehā a me ka lōʻihi o 200 mau minuke i kēlā me kēia pule.
Ua hōʻike ʻia nā hopena i hōʻemi ʻia ke kaumaha o ke kino o nā mea maʻi i mālama ʻia me ka semaglutide a me ka hoʻoikaika kino kino e 16% i hoʻohālikelike ʻia me ka baseline, aʻo ka hui placebo i hoʻemi ʻia e 5.7%.Mai ka ʻikepili o STEP3, hiki iā mākou ke ʻike i ka hopena o ka hoʻomaʻamaʻa a me ka meaʻai i ka pohō kaumaha, akā ʻo ka mea hoihoi, ʻo ka hoʻoikaika ʻana i ke ʻano o ke ola e like me ka liʻiliʻi o ka hoʻoikaika ʻana i ka hopena lāʻau o semaglutide.
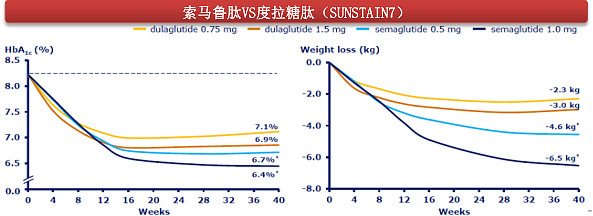
(Ka hoʻohālikelike o ka nui o ka pohō kaumaha ma waena o ka hui Semaglutide a me ka hui Dulaglutide)
Hiki i ka lāʻau lapaʻau ke hoʻonui i ka glucose metabolism ma o ka hoʻoulu ʻana i nā cell pancreatic β e huna i ka insulin;A ke kaohi ʻana i nā cell alpha pancreatic mai ka huna ʻana i ka glucagon, e hōʻemi ana i ka hoʻokē ʻai a me ke kō koko postprandial.
(Ka hoʻohālikelike o ke kaumaha o ke kino ma waena o ka hui mālama Semaglutide a me kahi placebo)
Ke hoʻohālikelike ʻia me kahi placebo, hiki iā Semaglutide ke hōʻemi i ka hopena o nā helu hope composite nui (ka make cardiovascular mua, nonfatal myocardial infarction, nonfatal stroke) e 26%.Ma hope o 2 mau makahiki o ka mālama ʻana, hiki i ka Semaglutide ke hōʻemi nui i ka hopena o ka maʻi maʻi ʻole e 39%, ka infarction myocardial non-fatal e 26% a me ka make cardiovascular e 2%.Eia kekahi, hiki iā ia ke hōʻemi i ka ʻai ʻana i ka meaʻai ma o ka hoʻohaʻahaʻa ʻana i ka ʻai a me ka hoʻolohi ʻana i ka ʻai ʻana o ka ʻōpū, a i ka hopena e hōʻemi i ka momona o ke kino, kahi e hoʻemi ai i ke kaumaha.
Ma kēia noiʻi ʻana, ua ʻike ʻia ʻo phentermine-topiramate a me GLP-1 receptor agonist i hōʻoia ʻia ʻo ia ka maikaʻi loa o nā lāʻau hoʻemi kaumaha ma waena o nā poʻe ʻoi aku ka momona a me ka momona.